What is Hydrogen Induced Cracking (HIC)?
Hydrogen-induced cracking (HIC), or hydrogen embrittlement, is a chemical phenomenon that causes metal alloys to fracture due to a build-up of hydrogen molecules within the crystal lattice structure. This unique mechanical failure model most commonly affects low alloys and high-hardness steel grades such as titanium (Ti). Often, components develop increased susceptibility to hydrogen-induced cracking due to the introduction of excess hydrogen molecules into the metal structure during forming or finishing processes.
In this article, you will learn what hydrogen-induced cracking (HIC) is, how to avoid hydrogen-induced cracking, detection tests, and repair methods.
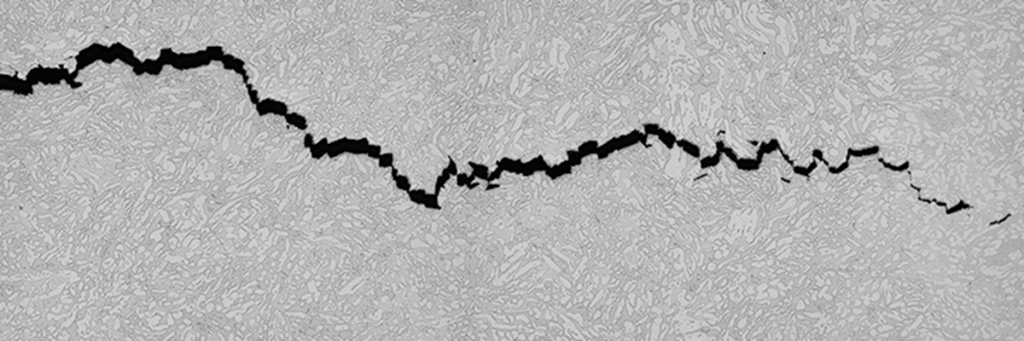
In regards to the amount of damage delivered, HIC usually, but not always, causes superficial damage. If HIC reduces ductility, the metal forms stepwise internal cracks. These cracks become dangerous should they propagate into welds.
How Does It Occur?
Hydrogen-Induced Cracking (HIC) occurs in carbon or low-alloy steel when atomic hydrogen diffuses into it and forms molecular hydrogen. Inclusions or trap sites may aid in the production of molecular hydrogen. As a result, HIC may occur when there is no stress. The creation of molecular hydrogen induces internal pressure, thus resulting in cracking.
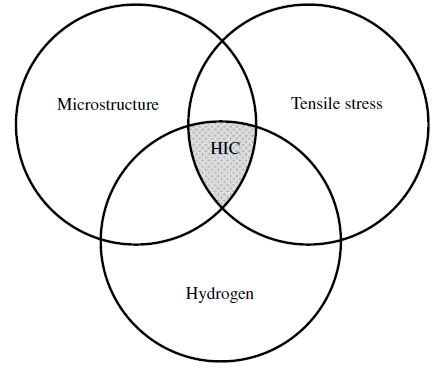
As a result, HIC also may be referred to as hydrogen pressure-induced cracking (HPIC). Impurities, inclusions, and microstructure (e.g., banding) created by segregation of impurity and alloying elements in the steel are all trap sites that cause HIC. Inclusion has a significant impact on HIC. During the rolling of steel, inclusions elongate and create voids. This increases the susceptibility to HIC.
How To Avoid Hydrogen-Induced Cracking (HIC)?
Prevention of hydrogen-induced cracking occurs by avoiding contact between the metal and any atomic hydrogen sources. Managing environmental conditions in potentially corrosive service is a must to avoid hydrogen ions from forming on the metal surface.
Carefully monitoring acid pickling and cathodic protection processes ensures hydrogen does not infuse at the component surface.

Some hydrogen absorption may be unavoidable during welding operations, depending on the welding method used. To restrict hydrogen absorption, the creation of extremely hard microstructures should be avoided. Also, hydrogen must escape before the workpiece reaches critical low temperatures. This requires careful control of welding conditions.
In certain processing activities, the absorption of a substantial amount of hydrogen may be unavoidable. In that case, avoiding embrittlement concerns occurs by a thermal exposure approach, sometimes known as “baking”. Baking allows hydrogen to escape before being exposed to low temperatures. Some types of equipment use shutdown processes and cooling control to enable hydrogen levels to reach required low numbers.
Another way for preventing cracking is to use materials that are less susceptible to hydrogen embrittlement. For materials that are not susceptible to SSC in hydrogen sulfide settings, the ISO 15156 Standard, for example, specifies hardness limits.
Detection Test of HIC
To avoid the risk of hydrogen corrosion, operators must regularly inspect and test for HIC.
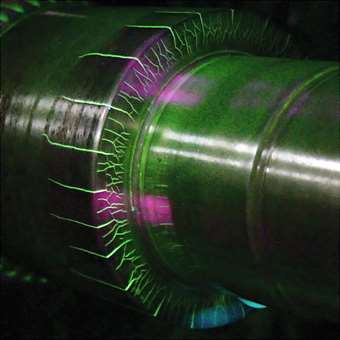
The common weld testing procedure known as Wet Fluorescent Magnetic Particle Inspection (WFMPI) identifies wet H2S cracking as it detects sub-surface cracks in steel induced by HIC. Fluorescent damp inspection detects very small discontinuities as it uses much smaller particles. The liquid carrier allows these particles to flow easily to small leakage fields to form indications.

When it comes to fractured piping and other components that don’t work with WFMPI, phased array ultrasonic testing (PAUT) provides the most convenient and reliable non-destructive procedure. Phased array ultrasonic testing provides a sophisticated type of ultrasonic testing and applies to a wide range of inspection activities. This technology finds complex faults using traditional non-destructive testing methods like radiography and manual ultrasonic testing.
Hydrogen-Induced Cracking (HIC) Repair Methods
An embrittlement relief or hydrogen bake-out cycle is a popular approach to reduce the hydrogen in the metal. This is an effective way to get rid of hydrogen before it causes damage to the part.
The bakeout should occur within 1 to 2 hours of introducing hydrogen to the material to be effective. Baking the hydrogen out of the part in an industrial oven at a specific temperature alleviates hydrogen embrittlement. In the aerospace sector, it is the preferred embrittlement alleviation approach.
Fasteners are one of the most prevalent aerospace components to suffer from hydrogen embrittlement. Electroplating fasteners absorb hydrogen, making the material brittle. Hydrogen embrittlement can also occur during pre-plating techniques, including cleaning and pickling, and during electroless plating.